Vaccine Information For Pregnant Women
No Pregnant Woman Died From Respiratory Illness and Women Vaccinated Against Flu Had The Same Risk For Flu As Unvaccinated Women
The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention recommends influenza vaccination for women who will be in the second or third trimester of pregnancy during the influenza season. We analyzed hospital admissions with principal diagnoses of influenza or pneumonia and influenza-like illness (ILI) outpatient visits to study the effectiveness of influenza vaccine during pregnancy in protecting women and infants from influenza-related morbidity. Estimates of influenza vaccine effectiveness across five flu seasons (Fall 1997 to Spring 2002) were calculated using Cox proportional hazards models for women and infant study populations in Kaiser Permanente Northern California. Outpatient utilization outcomes included physician visits with a diagnosis of upper respiratory infection, pharyngitis, otitis media, asthma, bronchial asthma, viral infection, pneumonia, fever, cough, or wheezing associated with respiratory illness. Inpatient outcomes included hospitalizations with principal diagnoses of influenza or pneumonia. Women who received influenza vaccine during pregnancy had the same risk for ILI visits compared with unvaccinated women, adjusting for women’s age and week of delivery. When asthma visits were excluded from the outcome measure, we also found no difference in the risk of outpatient visits for vaccinated and unvaccinated women. Hospital admissions for influenza or pneumonia for women in the study population were quite rare and no women died of respiratory illness during pregnancy. Infants born to women who received influenza vaccination had the same risks for influenza or pneumonia admissions compared with infants born to unvaccinated women, adjusting for infant’s gender, gestational age, week of birth, and birth facility. Maternal influenza vaccination was also not a significant determinant of risk of ILI (excluding otitis media) outpatient visits for infants, nor did it significantly affect the risk of otitis media visits. Influenza vaccination during pregnancy did not significantly affect the risk of cesarean section, adjusting for the woman’s age. It also did not affect the risk of preterm delivery. Although the immunogenicity of influenza vaccination in pregnancy in mother and infant has been well documented, in this study, we were unable to demonstrate the effectiveness of influenza vaccination with data for hospital admissions and physician visits. One possible interpretation of these findings is that typical influenza surveillance measures based on utilization data are not reliable in distinguishing influenza from other respiratory illness. Hospitalizations for respiratory illness were uncommon in both vaccinees and nonvaccinees.
Source: Am J Perinatol. 2004 Aug;21(6):333-9.
Vaccination During Pregnancy Does Not Prevent Respiratory Illness in Newborns
OBJECTIVE: To determine whether influenza vaccination of pregnant women prevents visits for respiratory illness in their infants born during the influenza season. DESIGN: Retrospective matched cohort study. SETTING: Four managed care organizations in the United States. Patients A total of 41 129 infants (3160 and 37 969 born to vaccinated and unvaccinated mothers, respectively) born between 1995 and 2001. Main Exposure Maternal influenza vaccination. Infants were considered exposed if their gestational age at birth was at least 30 weeks, if the time from maternal vaccination to birth was at least 28 days, and if they were exposed to at least 14 days of the influenza season. MAIN OUTCOME MEASURES: Incidence of acute respiratory illnesses (outpatient, emergency department, and inpatient settings combined) and incident rate ratios (IRRs) for infants exposed and unexposed to maternal vaccination during the following 4 periods: peak influenza, respiratory syncytial virus predominant, periseasonal, and summer weeks. The time to the first acute respiratory illness during peak influenza weeks was also assessed. RESULTS: During the peak influenza weeks, infant visit rates were 15.4 and 17.1 per 100 person-months for exposed and unexposed infants, respectively (IRR, 0.90; 95% confidence interval, 0.80-1.02). Adjusted IRRs for the 4 periods found a protective effect of infant female sex, whereas Medicaid status and maternal high-risk status increased infant visit rates. Maternal influenza vaccination did not reduce visit rates during any of the 4 time periods (IRR for peak influenza season, 0.96; 95% confidence interval, 0.86-1.07) and did not delay the onset of first respiratory illness. CONCLUSION: We were unable to demonstrate that maternal influenza vaccination reduces respiratory illness visit rates among their infants.
Source: Arch Pediatr Adolesc Med. 2006 Dec;160(12):1277-83.
Swine Flu Vaccine Can Damage The Brains of Unborn Babies
Source: Ground Report by Anne Hart, 22 October 2009.
Israeli Health Ministry DOES NOT Recommend Swine Flu Vaccine For Pregnant Women
Source: Haaretz.com, by Dan Even, 22 October 2009.
Miscarriages and Stillbirths After Swine Flu Vaccine!
Source: Prevent Disease.com, date not shown.
French Woman Loses Her Baby At 38 Weeks After H1N1 Vaccine
A French woman. a health care worker, has lost her baby at 38 weeks two days after taking the Pandemrix swine flu jab.
Source: Le Parisien, 20 November 2009 – reported by the flu case.

Pregnant Woman Paralysed By H1N1 Vaccine Suddenly Dies – Her Baby is Delivered By C Section, Alive
Source:
http://www.hs.fi/kotimaa/artikkeli/Aivokuolleen+naisen+vauva+pelastettiin+keisarileikkauksella+Kuopiossa/1135250982190
More True Life Accounts of Miscarriage and Stillbirth From Previously Pregnant Women
Source: http://organichealthadviser.com/archives/is-the-h1n1-swine-flu-vaccine-causing-miscarriages-9-new-stories-of-pain-and-loss-from-mothers-who-lost-their-babies-after-receiving-the-h1n1-vaccine
Doctors in Taiwan Say Pregnant Women Should Not Have H1N1 Vaccine In First Trimester
Source: Taiwan News, 14 January 2010.
Woman Has Stillborn Baby After H1N1 Vaccine
Source: The Standard, 21 January 2010.
Pregnant Women Warned Not to Get H1N1 Shot or Any Vaccine
Source: The Standard, 5 March 2010.
Pregnancy May NOT be a Risk Factor for H1N1 Flu
Source: Infectious Disease News, 15th September 2010.
Pano-Pardo J. V-652. Prognosis of 2009 A (H1N1) Influenza in Admitted Pregnant Women in a Context of Early Diagnosis and Therapy. Presented at: 50th Interscience Conference on Antimicrobial Agents and Chemotherapy; Sept. 12-15, 2010; Boston.
Newborn Dies after Mother was Vaccinated Against Flu in Pregnancy
Source: Irish Health, 29th February 2012.
Maternal thimerosal exposure results in aberrant cerebellar oxidative stress, thyroid hormone metabolism, and motor behavior in rat pups; sex- and strain-dependent effects
Source: Cerebellum. 2012 Jun;11(2):575-86 – http://www.ncbi.nlm.nih.gov/pubmed/22015705
Influenza Vaccination During Pregnancy: A Critical Assessment of the Recommendations of the Advisory Committee on Immunization Practices (ACIP)
Source: Journal of American Physicians and Surgeons Volume 11 Number 2 Summer 2006.
Full article here: http://www.jpands.org/vol11no2/ayoub.pdf
Embryonic exposure to thimerosal, an organomercury compound, causes abnormal early development of serotonergic neurons
Source: Neurosci Lett. 2011 Nov 14;505(2):61-4 – http://www.ncbi.nlm.nih.gov/pubmed/21669256
Maternal transfer of mercury to the developing embryo/fetus: is there a safe level?
Source:
Toxicological & Environmental Chemistry
Volume 94, Issue 8, 2012, http://www.tandfonline.com/doi/full/10.1080/02772248.2012.724574
Comparison of VAERS fetal-loss reports during three consecutive influenza seasons: was there a synergistic fetal toxicity associated with the two-vaccine 2009/2010 season?
Source: Hum Exp Toxicol. 2013 May;32(5):464-75. doi: 10.1177/0960327112455067. Epub 2012 Sep 27. http://www.ncbi.nlm.nih.gov/pubmed/23023030
Evaluation of the Association of Maternal Pertussis Vaccination With Obstetric Events and Birth Outcomes
Source: JAMA, November 12, 2014, Vol 312, No. 18
What is Chorioamnionitis?
Sources: JAMA, November 12, 2014, Vol 312, No. 18
Clin Perinatol. 2010 Jun; 37(2): 339–354.
http://reprolineplus.org/system/files/resources/MCHIP_Brief_LaborCarePrevNBSepsis_En.pdf
Pediatrician’s Warning About Vaccination in Pregnancy
Whooping Cough Vaccine Used in Pregnant Women Not Studied for Effect on Fetus
https://www.medicines.org.uk/emc/medicine/15256
Pregnant Woman Gets Pertussis from the DTaP Vaccine, Coughs for Last 3 Months of Pregnancy. Baby Gets Spinal Myxoma and Has to Have Back Surgery
Upregulation of TGF-beta 1 in neonates of mothers receiving Influenza A (H1N1) vaccination during pregnancy
Source: DOI 10.1111/all.12037
Official doubletalk hides serious problems with flu shot safety and effectiveness
After weeks of brooding about the Donahue article linking flu shots to miscarriages (Vaccine 2017;35:5314) it was with a sense of relief that I read Rob Wipond’s narrative of media attempts to sweep a serious vaccine safety issue under the rug….He points out the hypocrisy (his words were “double standard”) of authorities who dismissed the Donahue paper because it was an “observational study.” Year after year they have quoted observational studies to announce, “…80% vaccine effectiveness…60% effectiveness…40% effectiveness…” They do not mention that these studies make no effort to look for adverse vaccine effects (e.g. narcolepsy, seizures, high fever, oculorespiratory syndrome). They do not mention “negative vaccine effectiveness”, the increase in risk of illness from influenza and non-influenza viruses associated with (or caused by) the vaccines. (Cowling, Clin Inf Dis 2012;54:1778) They do not mention that a vaccine “effective” in one season may increase influenza risk in a subsequent season. (Read about “antibody-dependent enhancement” to understand one explanatory mechanism). They do not mention that the observational studies they refer to are likely to exaggerate vaccine effectiveness in the first place because of the “healthy user effect” well known to epidemiologists.
Full Commentary Here:
Source: BMJ 2018;360:k15
Influenza vaccination in pregnancy: careful assessment confirms safety concerns for the offspring.
Valid evidence does not support universal influenza vaccination for pregnant women, the LTE objections are unfounded. The observational evidence is less valid than that from RCTs: important safety signals in all the RCTs require high consideration. In RCTs, influenza vaccinated women have mostly local adverse effects, while their offspring shows a nonsignificant excess of deaths, and a significant excess of serious presumed/neonatal infections in the larger RCT. Several Authors have financial relationships with vaccine producers, several conclusions omit the safety signals. A cited systematic review has methodological problems and excluded important published RCTs. Waiting for new independent RCTs, the precautionary principle suggests avoiding to promote pregnant women vaccination. Health services could offer it highlighting existing uncertainties, with balanced informations allowing informed choices.
Source: Hum Vaccin Immunother. 2019 May 24:1-3. doi: 10.1080/21645515.2019.1605818. [Epub ahead of print]
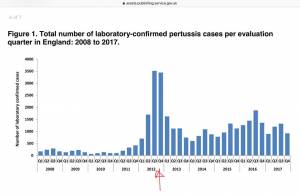
Whooping Cough Increases Since Vaccination in Pregnancy Introduced
In 2012, the UK began vaccinating all pregnant women against pertussis due to a lack of maternal immunity in vaccinated mothers and the resulting upsurge in pertussis in newborns. After this, cases of pertussis have increased.